A simple method of producing conductive nanomaterials may have uses from spacesuits to optoelectronics
Transparent electrodes, anti-radiation space suits and batteries that change color as they lose charge – all these may one day be manufactured with the help of a new class of self-assembling nanomaterials recently created at the Weizmann Institute of Science. These composite materials, made of carbon nanotubes and readily available organic (carbon-based) dyes, possess a unique combination of properties that may prove useful in optoelectronics, in colorful electrically-conductive devices and in separating carbon nanotubes from one another.
One remarkable property of the new nanocomposites produced in the lab of Prof. Boris Rybtchinski of the Organic Chemistry Department is the ease with which they can be created from simple molecular building blocks. Carbon nanotubes – exceptionally strong nanostructures made of carbon atoms – and organic dyes had been mixed together in the past, but this was usually done using complex dyes that contained additional constituents, so scientists had assumed that the outcomes were due to those additions. Now the Weizmann scientists mixed the carbon nanotubes in a solution with only a simple organic dye – and were thrilled by the results.

As reported in Advanced Materials, the mixing of just these two components sufficed to create a stable, conductive composite, in which the nanotubes were almost entirely separated from one another. This effect is of major significance in view of the fact that the tendency of nanotubes to stick to one another has greatly limited their performance in nanotechnology engineering. “We thought the mixing should work well, but we never expected such a dramatic effect. It was just marvelous,” Rybtchinski says.
Flexible, stable and colorful
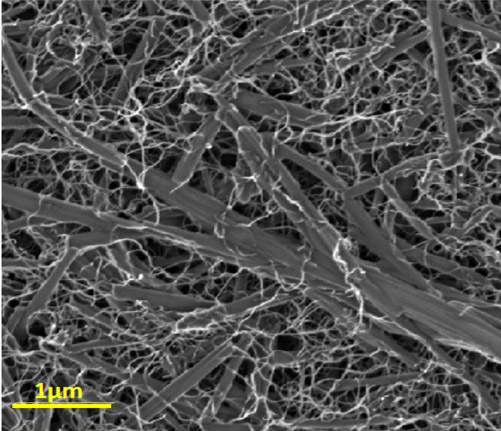
When the nanotubes are mixed together with the simple dye used in the study, the dye molecules “grab” electrons from the nanotubes, which leads to the formation of electrically charged molecular complexes. Rybtchinski and his colleagues discovered that in the wake of this transformation, the complexes became hydrophilic (“water-loving”), and this is what caused the nanotubes to separate in the solution. This is substantially cheaper and simpler than other existing methods of separating nanotubes. Moreover, the mixing improved the electrical properties of the nanotubes without interfering with their mechanical properties.
The hybrid nanocomposite itself – consisting of thread-like carbon nanotube structures and rod-like dye nanocrystals – could have numerous uses, because it combines the advantageous properties of its components. It is flexible, remains stable under heat and effectively conducts an electric current. Its colorfulness offers an extra benefit: The material could, for example, be used to paint the surface of cars or airplanes with a conductive or an antistatic coating of various colors, or to build batteries that signal a loss of charge by a change of color.

Thin films of the nanocomposite, when formed in organic or aqueous solutions, could be deposited on virtually any surface – for example, as an outer layer deflecting radiation from astronauts’ suits. Additional applications might stem from the fact that the nanocomposite contains numerous pores. Porousness creates a large surface area, which means that the material could be employed to manufacture extremely efficient electrodes. If the hybrid is deposited as an ultra-thin, transparent layer, it can help manufacture transparent electrodes suitable for use in the displays of various devices.
The research team included PhD student Angelica Niazov-Elkan, staff scientist Dr. Haim Weissman and postdoctoral fellow Dr. Sounak Dutta of the Organic Chemistry Department, as well as Drs. Sidney R. Cohen, Mark A. Iron, Iddo Pinkas and Tatyana Bendikov of the Chemical Research Support Department.
Prof. Boris Rybtchinski’s research is supported by the Solo Dwek and Maurizio Dwek Research School of Chemical Science, which he heads; and Dana and Yossie Hollander.

Recent Comments