Much as our earliest memories go into making our grown-up personalities, the cells in our bodies have “memories” that shape their identities. Cellular memories remind our skin cells to stay skin and bone cells to stay bone – even as these cells divide again and again. A team of scientists at the Weizmann Institute of Science recently investigated this cellular memory in cancer cells. Their findings may help us better understand the kinds of “memory loss” that help drive cancer.
Prof Amos Tanay of the Institute’s Mathematics and Computer Science, and Biological Regulation Departments explains that as a general rule, cancers start with genetic mutations, “but these can only partially explain the changes that cancer cells undergo as they divide and turn deadly.” Epigenetics – memory mechanisms that give our cells their identities by telling some genes to be expressed and others to be stably repressed – also get modified by cancer. This much was clear, but little else has been clear about if and how cancer cells change epigenetics to their benefit. For example, is this memory randomly lost or damaged in cancer, or could it be modified more directly as cells take on more cancerous features? Even if the process is random, Tanay wondered whether the deterioration of epigenetic memories would still push cancer forward, something in the way that random genetic mutations eventually conspire to turn cells deadly in the disease. Tanay and research student Zohar Meir decided to find out.

To probe cancer cell memory, Tanay and Meir developed a modern version of a classic elegant experiment. In the 1940s, Salvador Luria and Max Delbrück investigated a question about genetic mutations in bacteria. In the experiment, the scientists asked why, when bacteria are grown in the lab and challenged with viral infection, some cells can survive while others die. Did the cells have a “memory” of the infection that granted their progeny resistance? Luria and Delbruck grew colonies, each from a single bacterial cell, and after many generations they tested the colonies’ ability to survive a viral infection. The results were striking: Most of the colonies couldn’t resist viruses at all. But a small subset, each comprising millions of progeny all from the same bacterium, were nearly completely resistant. This led Luria and Delbruck — and soon after the entire new field of molecular biology – to understand some basic mechanisms of genetic heritability and to zoom in on DNA as the major carrier of genetic information.
Two generations later, in their lab at the Weizmann Institute of Science, the researchers grew cancer cells, rather than bacteria. They began with hundreds of single cancer cells and cultured each one of them separately, so as to obtain many distinct cancer colonies. To test how well these clones of single cancer cells retained their genetic and epigenetic memories, the team used new methods of single-cell sequencing. Luria and Delbrück had tested just one property for each of their colonies; in contrast, 21st century single cell genomics allowed Tanay and Meir to measure the RNA produced from thousands of genes over thousands of cells simultaneously, determining which gene expression programs are silenced and which are active in each cell and in each colony. As the cancer cells grew in the lab for six months, the team sequenced their RNA at several points along the way. By comparing earlier cells with their progeny, they could tell how closely the gene expression stuck to the original plan.
You can point to leaps, but in between, there are gradual changes that can nudge them in a more cancerous direction
“To our surprise,” says Meir, “we discovered a substantial component of epigenetic memory in cancer cells. The daughter cells inherit this memory almost as if it was their genetic identity. We actually had to carefully go back to the cells to make sure it was not their genetics – but the results were again very clear – what we found was truly non-genetic memory maintained through many cell divisions.”
In some of the cancer cells, taken from colorectal cancer, the researchers investigated the cells’ epigenetic makeup more closely, looking at DNA methylation – a series of tiny chemical tags that attach to the genes, helping prevent gene expression at particular points on the genome. Here, they found that as the generations of cancer cell clones reproduced, some of those tags fell off or moved, and these changes accumulated more rapidly than mutations in the DNA.
“The epigenetic memory is not entirely stable. It is something like a clock,” says Tanay. “The daughter cells may have only a few new mutations, even after many generations, but the methylation sort of slowly winds down, becoming less and less reliable. This slow drift in epigenetic memory can mean loss of control, as even a small percentage of those changes – removing the padlocks on certain genes so they can be expressed, for example – could help accelerate the cancer’s progression.”
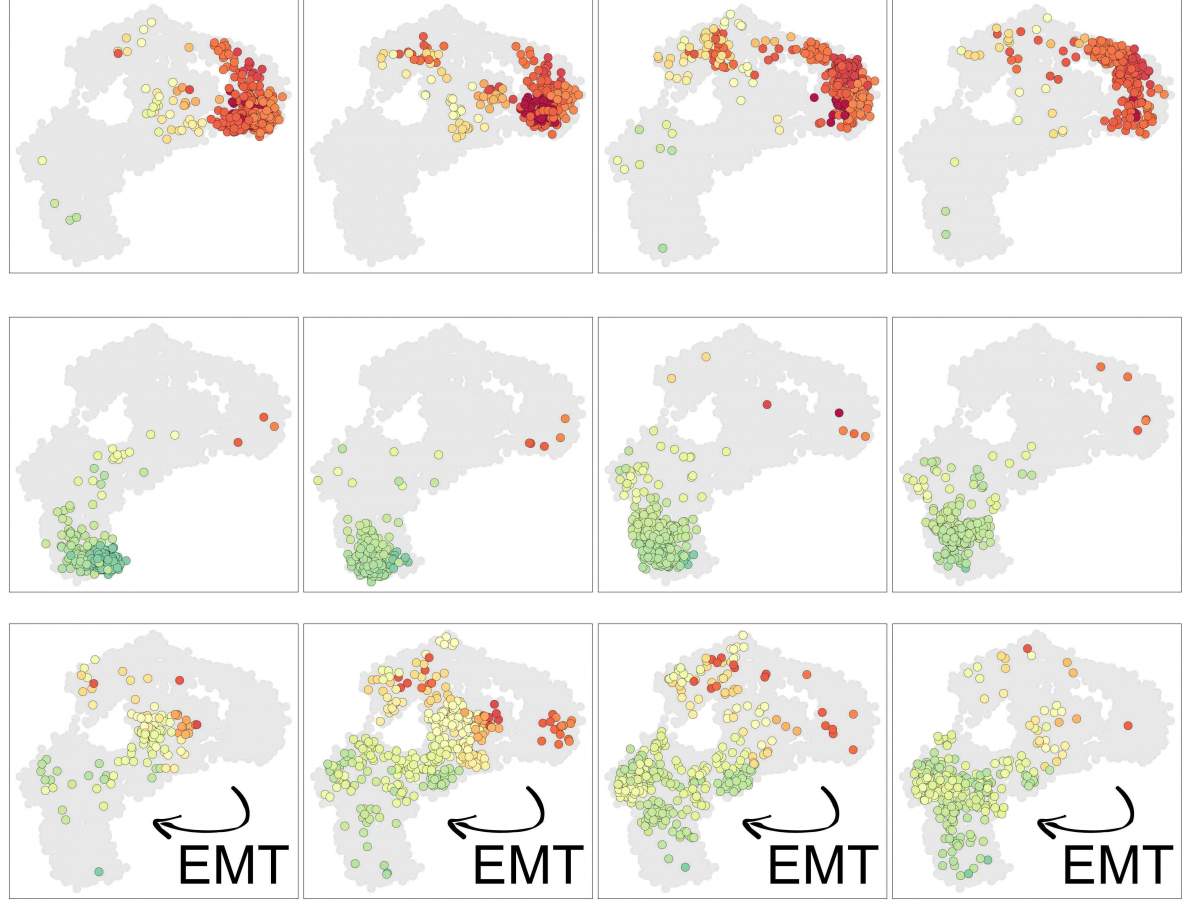
The researchers found that in some of these cells, the epigenetic memory loss maybe even helped the cells gradually forget which kind of cell they were meant to be, enabling them to transform into a different type of cell that can metastasize – a known phenomenon in cancer that had not been completely explained. “You can point to leaps in cancer progression that are tied to genetic mutation, but in between, there are gradual changes in the cells’ epigenetic makeup that can nudge them in a more cancerous direction,” says Tanay.
The scientists believe their findings point to new ways of better understanding the workings of epigenetic memory – in cancer and in healthy cells, as well. For example, their findings on the ticking of the epigenetic clock have implications not just for cancer, but for understanding the changes that take place in all of our cells as we age. Tanay and his team are already performing further experiments using the techniques developed in his lab to continue investigating how these memories function in cancer and other diseases.

Recent Comments